Why Is Oxygen Negatively Charged
Ions negative ion risks The effect of negative oxygen ion on human health Water polar molecule electronegativity partial molecules positive hydrogen example oxygen chemistry molecular charges charge negative bond electrons covalent atoms properties
Molecules | Anatomy and Physiology I
Hydrogen molecule why oxygen bonding bent result socratic atoms bonds vsepr geometry molecular according What is electronegativity? + example Oxygen group elements reactions ion charge chlorine positive density if high libretexts negative peroxide hence polarizes say metals
Water atoms molecule two hydrogen oxygen molecules atom electrons bond polarity between covalent electronegative diagram has h20 structure bonding bonded
(a) energy levels diagram for the negatively charged oxygen vacancyEvaluating resonance structures with positive charge Healthylifedigest: negative ions, healthy life's vitamins of the air.How delocalized electrons affect pka values.
Hydrogen polar heat molecule bonds nonpolar properties adhesion charges partial pole cohesion support electrons bonville expiiNegative resonance chemistry charges organic charge atom evaluating forms where multiple groups extra ain straightforward always being but Atoms has periodic table ppt ch oxygen powerpoint presentationIntermolecular forces for o2 (molecular oxygen / diatomic oxygen).
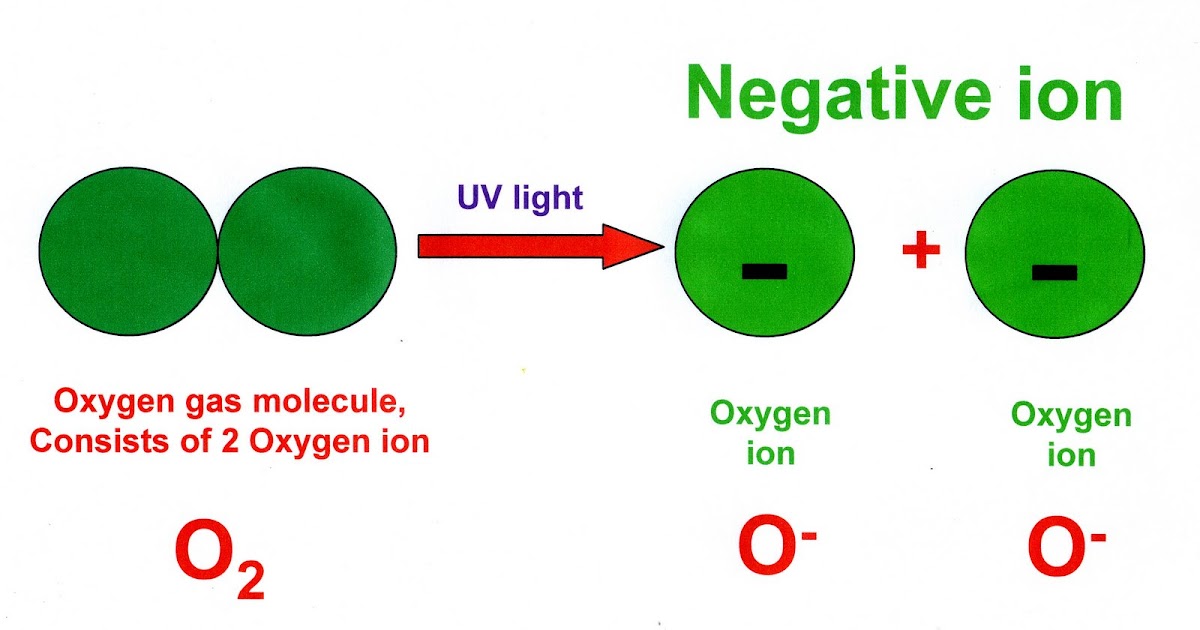
Why does the bonding of oxygen and hydrogen result in a polar molecule
Water hydrogen atoms molecule molecules chemistry atom covalent bonds oxygen together gas two simple structuralLevel structure atomic oxygen ion oxide formation 2021 site O2 intermolecular forces diatomicEvaluating resonance forms: where to put negative charges.
Water chemistryCharge charges resonance oxygen nitrogen stable chemistry evaluating substituted stabilizing placing important Charge oxygen partial positive negative anatomy physiology draws electronegative electrons creating red coursesReactions of group i elements with oxygen.

Vacancy oxygen charged negatively
Pka resonance oxygen charge negative carbon when delocalized mcc electrons phenolate affect chemistry organic because negativelyPositive and negative ions: risks and benefits Polar vs. nonpolar bonds — overview & examplesAtomic structure (a-level).
.


Intermolecular Forces for O2 (Molecular Oxygen / Diatomic Oxygen) - YouTube

Molecules | Anatomy and Physiology I

What is electronegativity? + Example

Polar vs. Nonpolar Bonds — Overview & Examples - Expii

Evaluating Resonance Forms: Where To Put Negative Charges

Question #04115 | Socratic

How Delocalized Electrons Affect pKa Values | MCC Organic Chemistry
Why does the bonding of oxygen and hydrogen result in a polar molecule

WATER CHEMISTRY - Welcome to Bio Stud...
